


Get the Facts on von Willebrand Disease
von Willebrand factor:
The main factor behind von Willebrand disease
The underlying cause of von Willebrand disease (VWD) is a deficiency or defect in von Willebrand factor (VWF), a multimeric protein important for clotting. Explore the pathophysiology and classification of VWD.1,2
VON WILLEBRAND DISEASE MECHANISM: VWF IS CRITICAL FOR HEMOSTASIS AND THROMBUS FORMATION AND PLAYS 2 KEY ROLES2,3:
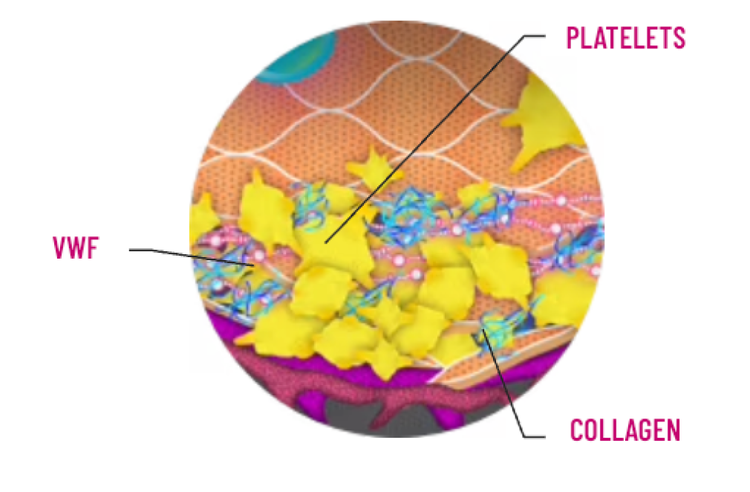

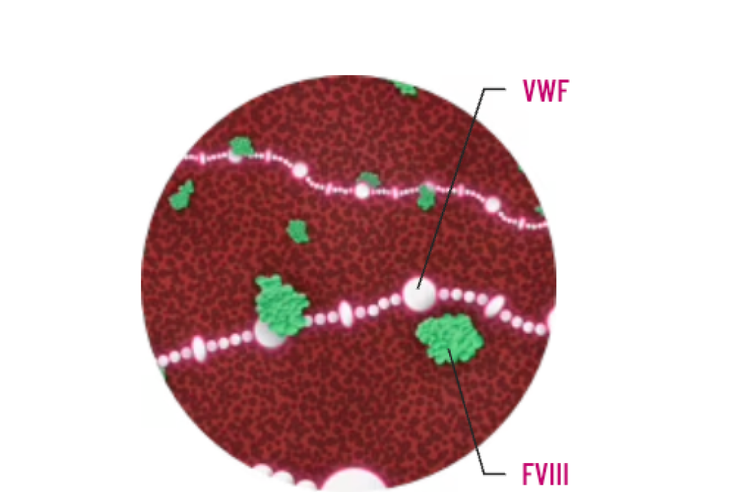

Because VWF protects FVIII in plasma, VWF deficiency or defect leads to increased FVIII clearance, resulting in low FVIII levels in some patients with VWD. Low levels of FVIII (<30-40 IU/dL) may lead to prolonged activated partial thromboplastin time (aPTT)2,4-6
There are 3 types of VWD7
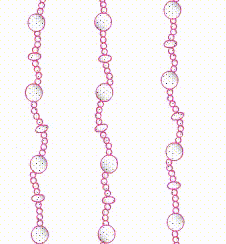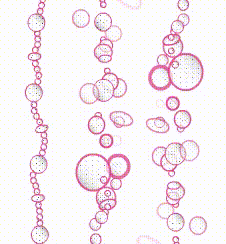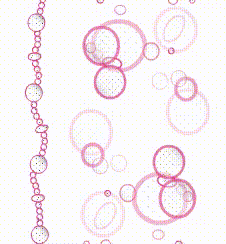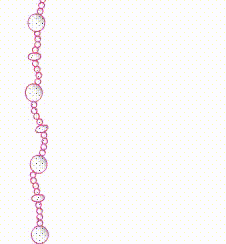
Type 1
VWF is deficient
Type 2
VWF is defective
Type 3
VWF is almost absent
Depending on type, VWD can be inherited in either an autosomal recessive or dominant manner.6
An important fact to note about all 3 types of VWD is that they only impact production of functional VWF—VWD itself does not impact patients’ ability to produce FVIII2,8-10
Bleeds happen in all types of VWD2
Because VWD patients lack VWF multimer protein, an important protein for clotting, they are prone to bleeds. Patients with VWD Types 1, 2, and 3 have all reported a range of bleeding episodes.

GINGIVAL

MENORRHAGIA

EPISTAXIS

GASTROINTESTINAL

JOINT
Severe Type 3 patients experience frequent bleeds
In an on-demand clinical trial where VONVENDI was being investigated, 21 adult patients with severe Type 3 VWD (VWF: Ag≤3%) experienced a median (range) of 5.7 (0.0, 36.5) bleeds in a year that required treatment.11
Could VONVENDI be right for your VWD patients?
VWF factor replacement can be used in 3 ways for patients with VWD12-15

On-demand
To treat acute bleeding episodes

Surgery
To manage bleeding around surgery

Prophylaxis (for adults only)
To proactively reduce the number of bleeds
2021 ASH/ISTH/NHF/WFH treatment guidelines have provided insight into when prophylaxis may be considered for recommendation in appropriate VWD patients.15
ASH=American Society of Hematology; ISTH=International Society on Thrombosis and Haemostasis; NHF=National Hemophilia Foundation; WFH=World Federation of Hemophilia.
Learn how VONVENDI works
See how VONVENDI is unique
Discover VONVENDI prophylaxis
For adults with VWD16
Contact a Takeda Rep
Get in touch with a Takeda representative in your area
References
- Gill JC, Castaman G, Windyga J, et al. Hemostatic efficacy, safety, and pharmacokinetics of a recombinant von Willebrand factor in severe von Willebrand disease. Blood. 2015;126(17):2038-2046.
- Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171-232.
- Turecek PL, Mitterer A, Matthiessen HP. Development of a plasma- and albumin-free recombinant von Willebrand factor. Hämostaseologie. 2009;29(suppl 1):S32-S38.
- Laffan M, Sathar J, Johnsen JM. von Willebrand disease: diagnosis and treatment, treatment of women, and genomic approach to diagnosis. Haemophilia. 2021;27 Suppl 3:66-74.
- Favaloro EJ, Pasalic L, Curnow J. Type 2M and Type 2A von Willebrand Disease: similar but different. Semin Thromb Hemost. 2016;42(5):483-497.
- James PD, Goodeve AC. von Willebrand disease. Genet Med. 2011;13(5):365-376. doi:10.1097/GIM.0b013e3182035931
- The Lewin Group. Strategic Summit on Von Willebrand Disease. National Hemophilia Foundation. March 2015.
- Leebeek FWG, Peyvandi F, Tiede A, et al. Diagnosis and treatment of von Willebrand disease in 2024 and beyond. Eur J Haematol. 2023:1-12. doi:10.1111/ejh.13999
- Borràs N, Batlle J, Pérez-Rodríguez A, et al. Molecular and clinical profile of von Willebrand disease in Spain (PCM-EVW-ES): comprehensive genetic analysis by next-generation sequencing of 480 patients. Haematologica. 2017;102(12):2005-2014.
- de Wee EM, Sanders YV, Mauser-Bunschoten EP, et al. Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108(4):683-692.
- Takeda Data on File. Severe Type 3 Bleeds
- Laffan MA, Lester W, O’Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol. 2014;167(4):453-465.
- Berntorp E, de Moerloose P, Ljung RC. The role of prophylaxis in bleeding disorders. Haemophilia. 2010;16(suppl 5):189-193.
- Saccullo G, Makris M. Prophylaxis in von Willebrand disease: coming of age? Semin Thromb Hemost. 2016;42(5):498-506.
- Connell NT, Flood VH, Brignardello-Peterson R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301-325.
- VONVENDI [von Willebrand factor (Recombinant)] Prescribing Information.



